Istesso Limited (“Istesso’’) today announces headline results from the Phase 2a study of its investigational drug, MBS2320, in rheumatoid arthritis. The study was a randomised, double-blind, placebo-controlled trial for which the primary objective was to assess the safety and tolerability of MBS2320 over 12 weeks of treatment in patients receiving background methotrexate (MTX) therapy. The primary objective was met: MBS2320 was generally well-tolerated with no drug-related serious adverse events (SAEs).
Amongst secondary objectives, there was evidence of clinical benefit as assessed by the American College of Rheumatology 20% response (ACR20) and Disease Activity Score (DAS28-CRP). Evidence of benefit was also seen in exploratory endpoints of responder criteria, MRI imaging and the acute-phase reactant, C-reactive protein (CRP).
For further information, please contact:
Istesso Limited
Sam Williams, Executive Chairman +44 207 444 0066
Notes to Editors
About rheumatoid arthritis
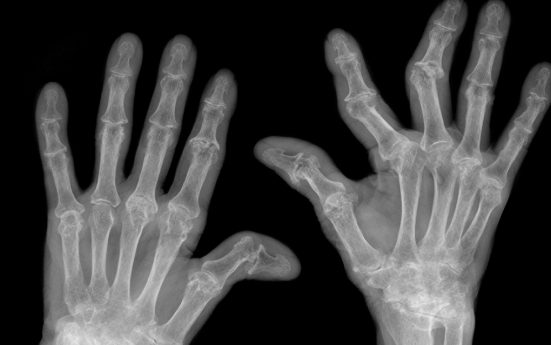
Rheumatoid arthritis (RA) is a chronic, progressive and disabling auto-immune disease affecting 165 million people worldwide, or one per cent of the global population. It is a painful condition that, unchecked, can cause severe disability. The disease can progress very rapidly, causing swelling and damaging cartilage and bone around the joints. Any joint may be affected but the hands, feet and wrists are most commonly involved, preventing patients from carrying out everyday tasks.
About MBS2320
MBS2320 is a first-in-class metabolic reprogramming agent for the treatment of RA and is distinguished from existing treatments by its dual mode-of-action which not only reduces inflammation, thereby preventing the progression of disease, but may also promote bone and joint remodelling.
About Istesso
Istesso is a drug discovery and development company working in the field of immunometabolism. Istesso’s products work by reprogramming cellular metabolism and have applications in autoimmune conditions such as rheumatoid arthritis and multiple sclerosis, as well as cancer. For more information please visit www.istesso.co.uk.
ENDS